Diagnostic’s ALS test
Background
Iron Horse Diagnostics was founded by Dr. Robert Bowser, who was awarded the 2015 Sheila Essey Award by the The American Academy of Neurology and The ALS Association for his work in the diagnosis of ALS. Dr. Bowser has been researching ALS for over 20 years; first at the University of Pittsburgh and more recently at the Barrow Neurological Institute.
Dr. Bowser developed the protein signatures for both the ALS diagnostic test and the ALS prognostic test. Dr. Bowser has published over 100 articles in peer-reviewed journals and has been the recipient of a large number of grants for his research in ALS. Dr. Bowser is also a Professor of Neurology and Neurobiology at the Barrow Neurological Institute and St. Joseph’s Hospital and Medical Center in Phoenix, Arizona. In addition, Dr. Bowser is Director of the Gregory W. Fulton ALS
Research Center in Phoenix, Arizona.
Iron Horse is offering a simple, quick blood-based screening assay and a secondary CSF-based confirmatory diagnostic test, as well as prognostic tests to be used to monitor disease progression. This portfolio of diagnostic and prognostic tests for ALS fills a very large unmet need as currently there is no simple and cost-effective way to diagnose this deadly disease.
Clinical Significance
Our diagnostic has already been tested in thousands of patients. Iron Horse and our European Licensee Euroimmun have tested over 750 patient samples, Independent researchers have tested our biomarker on over 871 patients and Iron Horse, acting as a CRO, has tested over 1,000 samples for various entities. Euroimmun began offering kits to laboratories in Europe in 2018 and the assay has been utilized on thousands of patients.
Biomarkers
Iron Horse Dx utilizes two biomarkers in our assay. The first biomarker is (“pNFH”) neurofilament heavy chain, which has been determined to be the gold standard for ALS diagnosis by independent researchers. The second biomarker is Complement C3. Our assay has been optimized over 7 years of testing.
Plasma
Our current blood-based diagnostic test has 83% sensitivity and 86% specificity (accuracy = 84%) in predicting ALS. The availability of a simple blood test for general physicians to use as an initial investigation into patient symptoms would be of great value, both in terms of time and cost, to quickly identify those patients who have a neurologic condition and may have ALS to quickly refer to a neurology specialist. Think of it as a rapid screening test to identify patients at risk to be followed by our more accurate CSF test to confirm the diagnosis.
Cerebrospinal Fluid
The CSF based diagnostic test is 92% ROC accurate (94% sensitivity and 86% specificity at predicting ALS.
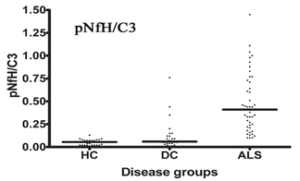
Figure 1 shows ALS diagnostic data for the CSF by measuring pNFH and complement c3 levels. Elevated ratio of these two proteins is evident in ALS patients versus healthy control (HC) or other neurologic disease (DC) subjects. The DC group included ALS disease mimics. A total of 126 subjects were used for this analysis. The overall accuracy of our ALS diagnostic test was 92% in this study.
Other Independent Research using Iron Horse Biomarker for ALS
Neurofilament heavy chain has been tested by numerous independent researchers and has been determined to be the biomarker of choice for ALS diagnosis. According to Meta-Analysis documented in Frontiers in Aging Neuroscience titled, “Neurofilaments in CSF as Diagnostic Biomarkers in Motor Neuron Disease: A Meta-Analysis”, they concluded that Neurofilaments in CSF have a high value in the diagnosis of Motor Neuron Disease.
ALS Prognostic Assays
Disease progression and determination of survival time can be obtained using samples collected in a longitudinal manner over time during the course of the disease. Information from these prognostic assays is very important to monitor drug effects in clinical trials or identify patient sub-populations that may best be treated with particular drug or drug combinations.
In one study, we examined our biomarkers in longitudinal samples from more than 20 ALS patients taken every 3 to 4 months over a 2 to 3 year time period and compared the results to matching information regarding clinical markers of disease progression. The levels of pNFH increase very early in the disease and we have a number of examples of individuals positive for ALS with our diagnostic before a clinician could detect the disease. The pNFH levels remain constant over time during disease progression, differ between ALS patients and correlates with survival time. Therefore, we can predict survival of patients based on the level of pNFH in blood or CSF. Consequently, our assays can be used to help determine if a drug is beneficial to the patients as part of a clinical trial or with existing therapies currently on the market.
Uses
The Iron Horse ALS tests are designed to accelerate the early diagnosis of ALS and to differentiate ALS from ALS disease mimics. Earlier diagnosis is key to starting therapeutic regiments to enhance the patient’s quality of life.
We believe that for patients that exhibit any symptoms of ALS, the pre-screening plasma assay can give the physician a quick look at the possibility of ALS, while for those that exhibit more advanced symptoms, the CSF confirmatory assay can be used. For patients that have already been diagnosed with ALS, the prognostic tests can be used throughout the treatment process to monitor disease progression and treatment effectiveness.